While commonly associated with muscle fatigue and fermented food products, lactic acid is a highly industrial organic compound that is utilized in different industries. As a food additive, lactic acid is an effective acidulant and flavor enhancer that can help provide food products with a mild acidic and tangy flavor. Its low pH can also regulate the acidity of the food products to eliminate residual microorganisms and perform as an effective preservative.
Lactic acid is most likely vegan. Most of the lactic acid industrially produced comes from fermentation using lactic acid bacteria. Most substrates used for fermentation are starchy carbohydrates. However, there are some companies that produce lactic acid using dairy products such as lactose and whey.
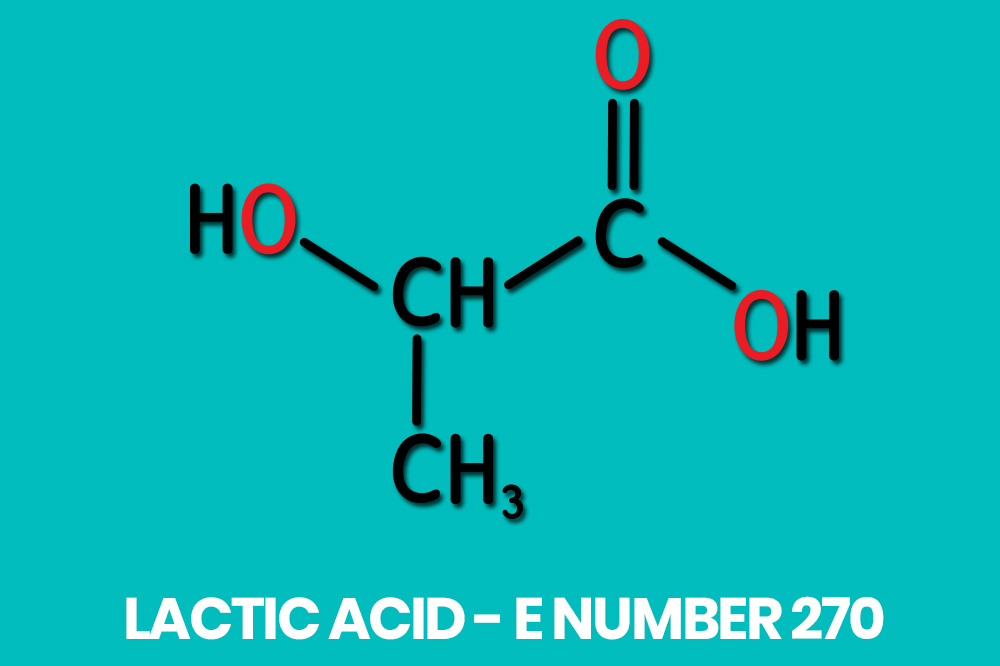
Lactic acid is an organic compound denoted by the chemical formula C3H6O3. It is also known as 2-hydroxypropanoic acid and can be found listed in some ingredients list by its E number, E270.
Lactic acid was first discovered in sour milk back in 1780. Originally, it was thought to be a component of milk (1). It took a few decades before it was determined that lactic acid was not a component in milk but in fact a byproduct of milk fermentation produced by certain microorganisms. After a few more decades of research, it was not until 1881 before lactic acid was first commercially produced.
Aside from being produced by microorganisms, lactic acid can also be produced by plants, animals, and even humans.
Most notably, many might be familiar with lactic acid and how it is involved with muscle fatigue. During times of intense exercise, the muscles begin to quickly deplete the available oxygen in the surrounding environment. At this point, the muscles begin to utilize anaerobic respiration for additional energy. Although anaerobic respiration can produce energy without oxygen, the pathway produces lactic acid as a byproduct. Eventually, the buildup of lactic acid acidifies the muscular environment which subsequently leads to muscle fatigue and soreness (2).
Structurally, lactic acid can exist between either of its two optical isomers: D-lactic acid and L-lactic acid. However, L-lactic acid is the preferred isomer used in the food and pharmaceutical industries as studies have shown that elevated levels of D-lactic acid can be harmful to humans.
Industrially, lactic acid can be found effectively utilized in a number of industries including leather, textiles, and even the production of biodegradable and biocompatible polylactate polymers, such as polylactic acid. From a global lactic acid demand of 714.2 kilo tons in 2013, estimates have shown that lactic acid demand grows annually by about 15.5% (3).
The industrial applications of lactic acid are quite varied. Estimates show that most of the lactic acid produced goes to the polymers (39%) and food and beverages (35%) industries. The remaining uses of lactic acid are for personal care (13%) and solvents and other industrial uses (13%).
In the food industry, lactic acid is specifically used for a number of reasons. Due to its mild acidic taste, lactic acid is commonly used as an acidulant. It is acidic enough that it can also be used as a preservative. The food additive can also be used as a flavoring agent, pH regulator, and even as an inhibitor of residual microorganisms in food processing.
Due to its history and discovery, some people worry that lactic acid is produced from milk. However, most lactic acid produced today comes from the fermentation of lactic acid bacteria using carbohydrate substrates such as glucose, corn starch, cassava starch, sweet sorghum, and other plant sources.
Thus, the lactic acid found in food products is most probably suitable for vegans.
However, lactic acid cannot be totally declared vegan because there are instances where non-vegan substances can be used. Although less common, there are manufacturers that utilize non-vegan substrates such as lactose and whey.
This uncertainty might be enough for vegans to avoid products with lactic acid, but lactic acid can be considered a gray area at best. It is to be emphasized that while it is possible to produce lactic acid using non-vegan substrates, most lactic acid produced should be perfectly vegan. The final verdict is up for the individual’s discernment.
Having two optical isomers, companies can produce lactic acid in either conformation or in mixtures containing varying proportions of both isomers. However, pure isomers have greater value than isomeric mixtures because pure isomers can be used for specific industrial applications.
There are two main ways to produce lactic acid: chemical synthesis and microbial fermentation.
Through chemical synthesis, the starting substrates needed are typically petrochemical resources. From petrochemical resources, acetaldehyde is obtained. The acetaldehyde is then reacted with hydrogen cyanide to produce lactonitrile. Lastly, the lactonitrile is hydrolyzed with a strong acid such as hydrochloric acid or sulfuric acid to produce lactic acid.
However, chemical synthesis of lactic acid can only produce the isomeric mixture of both lactic acid isomers – not pure isomers. This is one reason why modern lactic acid production is predominantly (~90%) through microbial fermentation.
Biotechnologically, lactic acid can also be derived from bacteria – just as how it was discovered before. Beginning with a carbohydrate substrate such as glucose, the fermentation process simply inoculates lactic acid producing bacteria into a fermentation tank filled with a growth medium that can be converted to lactic acid. Many bacteria can be used for fermentation, but industries typically use bacteria from the genera Lactobacillus, Lactococcus, and Bacillus.
The quality of the resulting lactic acid produced through fermentation is significantly better when the substrate has high purity such as sucrose from sugarcane and sugar beet. Using these substrates can reduce the cost of lactic acid purification.
However, since pure substrates can become quite costly, some industries would prefer industrial wastes as substrate. Waste can come from food industries, agricultural industries, sugarcane mills, and more. While purification might cost more in the long run, using industrial wastes as a substrate is advantageous from an environmental and economic standpoint.

As a food additive with many purposes, lactic acid can be found in many food products. These can include pickled vegetables, sourdough bread, beer, wine, kimchi, sauerkraut, and fermented soy products like soy sauce and miso.
Lactic acid is also present in other fermented products such as kefir and yogurt. As a fermented type of meat, salami can also contain lactic acid.
Safety is always a priority when it comes to substances produced for human consumption. Using numerous safety studies and internal standards, lactic acid has been reviewed by various food safety authorities and the general consensus is that the food additive poses no concern to the general public.
The JEFCA (Joint FAO/WHO Expert Committee on Food Additives) has evaluated lactic acid back in 2001 (4). Recognizing the use of lactic acid in the food industry as a flavoring agent and acidity regulator, the JEFCA does not require an acceptable daily intake value (ADI; the maximum amount of a substance an individual can consume daily for a year without any adverse health effects) and believes that the food additive does not pose any safety concern at current levels of intake.
The FDA (Food and Drug Administration) recognizes that lactic acid meets the specifications of the Food Chemicals Codex and can be used without limitation other than good manufacturing practice (5). Specifically, the FDA allows the use of lactic acid as an antimicrobial agent, a curing and pickling agent, a flavor enhancer, a flavoring agent and adjuvant, a pH control agent, and a solvent and vehicle.
The only area where lactic acid is specifically described to be quantifiably controlled is in infant food and infant formula. Overall, the FSA categorizes lactic acid as GRAS (“generally recognized as safe”).
References
