Malic acid is a naturally occurring organic compound that can be found in many fruits and vegetables. It is also a common food additive used in many products, typically serving the role of a flavor enhancer. While some products are distinctly sweet, malic acid is used to provide products with pleasant sourness and tartness that are typically associated with fruit flavors.
This organic compound is vegan as it is produced without the use of any animal product or derivative. While it can be found in natural sources such as fruits and vegetables, malic acid is typically industrially produced using maleic anhydride or fumaric acid – both vegan materials as well. While malic acid in food and pharmaceuticals is perfectly suitable for vegans, malic acid used in the cosmetic industry might not be ethically vegan.
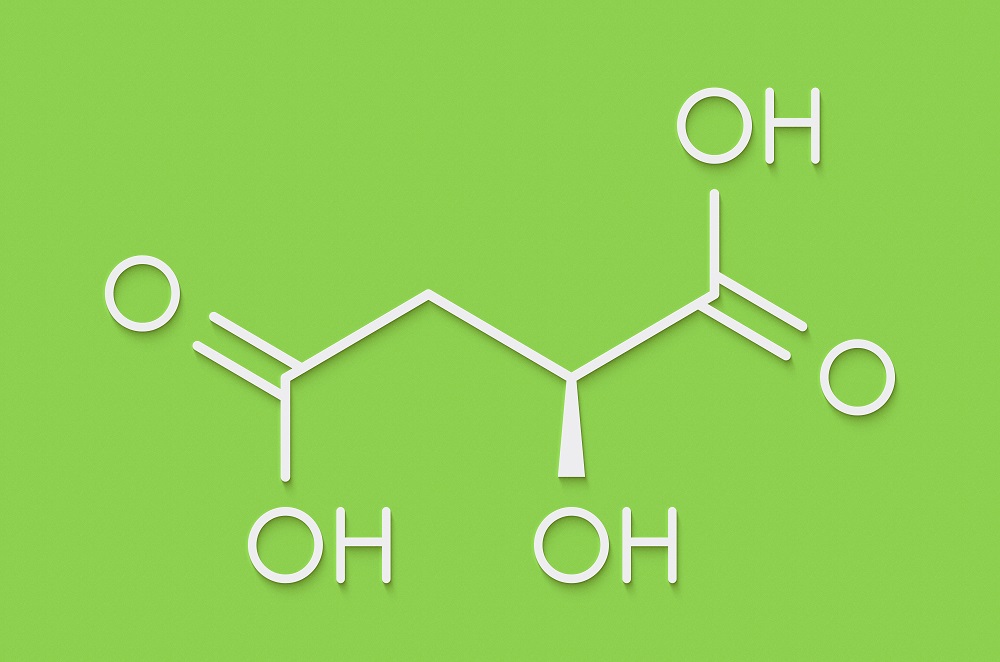
Malic acid is an organic compound that is heavily used in the food industry as an acidulant and flavor enhancer. It is denoted by the chemical formula C4H6O5 and it derives its name from where it was first isolated from: apples (genus Malus). While typically listed as malic acid in ingredients lists, it can also be listed by its E number, E296.
Malic acid is a naturally occurring substance in all aerobic organisms since it is an intermediate in the tricarboxylic acid cycle (TCA; also known as the citric acid cycle and the Krebs cycle). This biochemical pathway is crucial as it generates many products such as amino acid precursors and the molecular currency NADH.
Malic acid can exist in two forms: L- and D-malic acid. While only the L-malic acid can be found in nature, the organic compound is typically produced as a racemic mixtures – a quantity equally composed of both L- and D-forms.
The organic compound is especially abundant in several fruits. Aside from apples, malic acid is also found in large quantities in apricots, blueberries, blackberries, grapes, peaches, pears, quinces, plums, and cherries.
Malic acid serves different roles in different products, but the organic acid is primarily used as a flavor enhancer. It is an ideal flavor enhancer because when malic acid is used in products, fewer flavor additives are needed thus providing a broader flavor profile while simultaneously improving the production economy.
The preferred use of malic acid can also be attributed to its chemical properties. Malic acid is very soluble in water and also exhibits a lower melting point – two qualities that are highly ideal for products to achieve longer shelf-lives.
Malic acid is also used as a precursor to salts and esters that are also used as food additives. These malates include calcium malate, magnesium malate, and zinc malate. While these food additives can be used as flavorings and processing aids, they are often used as nutritional supplements to add more minerals to food products (1).
While malic acid can be found in many natural sources, it is conventionally produced for the food industry through chemical and enzymatic routes. The production does not involve any animal product or derivative which means malic acid is vegan.
While malic acid production is free from animal exploitation, some vegans might be uncomfortable with the product as it has been documented in some animal testing studies, particularly for the cosmetic industry (2).
The cosmetic industry has been associated with rampant animal testing studies due to the safety requirements imposed by legal and industrial standards. While the studies found malic acid to be safe for consumption, it was found to be a strong skin and eye irritant in the animal test subjects.
Animal testing is considered to be an unethical practice as it subjects unethical conditions to test animals. Standing for animal rights, many vegans abstain from products that undergo animal testing.
Due to the relative simplicity of its chemical structure and its abundance in nature, malic acid can be potentially produced through chemical, enzymatic, and biotechnological methods. However, the bulk of malic acid used in the food and pharmaceutical industries is typically from chemical and enzymatic means (3).
As the dominant method of producing malic acid, the chemical synthesis of malic acid uses maleic anhydride as its raw material. Exclusively obtained through fossil resources (particularly n-butane), maleic anhydride is hydrated in an aqueous solution to maleic acid.
The maleic acid produced from the hydration undergoes isomerization to become fumaric acid which is finally hydrated to malic acid.
Depending on many factors (e.g., resources, purity, etc.), some industries would prefer to produce malic acid enzymatically.
Through the enzymatic process, fumaric acid is converted to malic acid through hydration using the enzyme fumarase. The enzyme is typically obtained from microorganisms such as Saccharomyces cerevisiae, Brevibacterium flavus, Brevibacterium ammoniagenes, or Rhizopus oryzae.
Since malic acid is naturally found in nature, many natural food items would contain this organic compound. However, some products would have higher malic acid content than others.
Examples of fruits and vegetables that would have a considerable amount of malic acid include apples, peaches, watermelons, lychees, bananas, blackberries, corn, pears, broccoli, carrots, peas, and tomatoes.
As a food additive, malic acid can also be found in candies, chewing gum, fruity desserts, soy yogurt, carbonated sodas, iced tea, fruity drinks, and wine.
Malic acid is generally considered to be safe and is certified by various food safety authorities.
The FDA (Food and Drug Administration) declares that malic acid has passed the specifications of the Food Chemicals Codex, thereby categorizing the food additive as GRAS (generally recognized as safe) (4).
According to the FDA, malic acid is safe for consumption when used as a flavor enhancer, flavoring agent and adjuvant, and pH control agent.
Furthermore, malic acid is bound to good manufacturing practice and at specific limits (i.e., 3.4% in nonalcoholic beverages, 3.0% in chewing gum, 0.8% in gelatins, puddings, and fillings, 6.9% in hard candies, 2.6% in jams and jellies, 3.5% in processed fruits and fruit juices, 3.0% in soft candies, and 0.7% in all other food categories).
Pure malic acid can cause severe skin irritations, but the concentrations typically found in food are of little concern.
References
1. https://efsa.onlinelibrary.wiley.com/
2. https://journals.sagepub.com/
